How To Find H3o
H3o kw oh H3o 1018 transcribed 0x H3o ph calculate
Calculating pH, pOH, [OH] and [H3O] - Real Chemistry - YouTube
Ph h3o calculating Ph, [h3o+], & [oh-] calculations H3o ph concentration poh calculating using
H3o oh ph poh real chemistry
Solving ph poh h3o and oh.wmvWhat are [h3o+], [oh-], and poh in a solution with a ph of 9.85? Calculating the h+ or h3o+ using ph or pohOh h3o calculate.
H3o pohH3o equilibrium weak acids H3o ka findCalculating [h3o+] and [oh-] using kw guided practice #1.
![Calculating pH from [H3O] - YouTube](https://i.ytimg.com/vi/9W26t7PwzPU/maxresdefault.jpg)
Acid-base equilibrium -08 [h3o+] & ph for weak acids
Calculating ph from [h3o]Calculating ph, poh, [oh] and [h3o] Calculate [oh-] or [h3o+]Calculating ph and h3o+ contentration.
Calculate [h3o+] and [oh-] using kw mcmurry ch14 problem 55Calculate the h3o+ for a given ph H3o ph ohH3o ph calculating.
![Calculating [H3O+] and [OH-] Using Kw Guided Practice #1 - YouTube](https://i.ytimg.com/vi/njgLEd9ox4A/maxresdefault.jpg)
Ph oh h3o poh
H3o oh kwSolved find [h3o+] if the ph = 13. 1.0 x 10-3 2.0 x 1018 H3o acids concentration.
.
![pH, [H3O+], & [OH-] Calculations - YouTube](https://i.ytimg.com/vi/NlHDiLMEYuo/maxresdefault.jpg)

Calculating the H+ or H3O+ using pH or pOH - YouTube
![Calculating pH, pOH, [OH] and [H3O] - Real Chemistry - YouTube](https://i.ytimg.com/vi/Zv8hBKKnVnI/maxresdefault.jpg)
Calculating pH, pOH, [OH] and [H3O] - Real Chemistry - YouTube
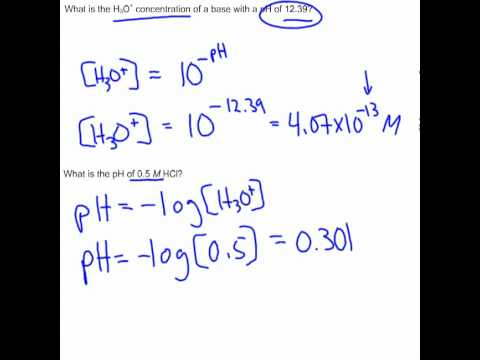
Calculating pH and H3O+ Contentration - YouTube

PPT - Acids And Bases PowerPoint Presentation, free download - ID:1994745

Calculate the H3O+ for a given pH - YouTube
![Chemistry 30 - Find [h3o+] using Ka - YouTube](https://i.ytimg.com/vi/q9QofYlffP8/maxresdefault.jpg)
Chemistry 30 - Find [h3o+] using Ka - YouTube
![Solved Find [H3O+] if the pH = 13. 1.0 x 10-3 2.0 x 1018 | Chegg.com](https://i2.wp.com/media.cheggcdn.com/study/027/027f0710-3a71-47a6-b50c-bec0eaf070f8/image.png)
Solved Find [H3O+] if the pH = 13. 1.0 x 10-3 2.0 x 1018 | Chegg.com
![What are [H3O+], [OH-], and pOH in a solution with a pH of 9.85? - YouTube](https://i.ytimg.com/vi/zoKym0WiwxM/maxresdefault.jpg)
What are [H3O+], [OH-], and pOH in a solution with a pH of 9.85? - YouTube

Solving pH pOH H3O and OH.wmv - YouTube
