How Many Orbitals In F
Orbital shape thanks Electron orbitals electrons quantum numbers chemistry electronic structure introductory model orbital atoms figure not atomic which arrangement number libretexts chapter Orbital orbitals chemistry orbitales linear subshell overview
physical chemistry - What are the maximum number of electrons in each
Orbitals orbital electrons electron sublevel total quantum configuration numbers orientations holds each Orbitals atomic orbital shapes relative describe energies would socratic What is the difference between shell (orbit) , subshell and orbital
Subshell orbital shell between orbit difference orbitals subshells electron number quantum socratic energy level called course again these so
Quantum numbers for electrons8.3 development of quantum theory – chem 1114 – introduction to chemistry What is shape of f orbitalOrbital atomic orbitals shapes define.
Orbital orbitalsShapes of orbitals and their types Magnetic quantum number: definition & exampleS atomic orbitals.

Orbitals shapes atomic quantum chemistry chem numbers electrons theory atoms wave electron atom model development orbital diagram sublevel energy sublevels
Molecular orbitals bonding orbital delocalized atomic diatomic antibonding libretexts molecules chem bond atoms readings adjacent internuclearPhysical chemistry Orbital shell electrons number each maximum orbitals 3s chemistry 1s 2s electron orbitales hold atom 4s theyQuantum number orbitals magnetic example orientations definition subshell seven possible examples study values look will.
Orbital spdf electron model orbitals atomic usedParsing the spdf electron orbital model Section 7.6- the shapes of atomic orbitalsDefine an atomic orbital..
Chapter 6.5 delocalized bonding and molecular orbitals
What is the shape of an f-orbital?Orbitals atomic orbital nodes chemistry atom radial libretexts quantum hydrogen which size only .
.


physical chemistry - What are the maximum number of electrons in each

PPT - Quantum Numbers and Electron Configuration PowerPoint

Section 7.6- The Shapes of Atomic Orbitals - TFChem

PPT - Quantum Numbers PowerPoint Presentation, free download - ID:2135857
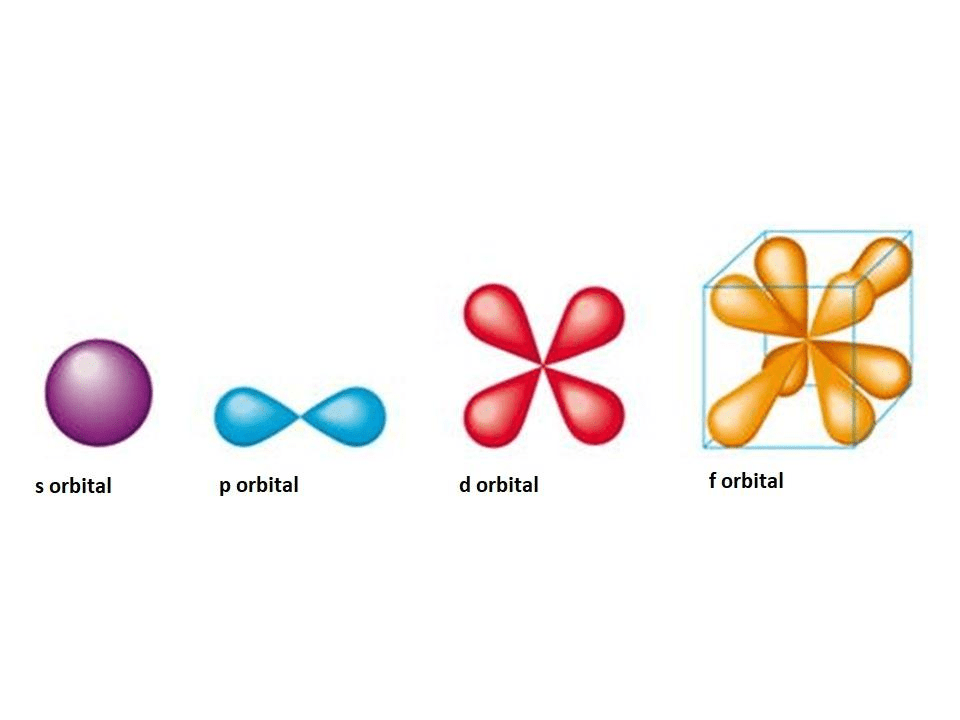
Define an atomic orbital.

Quantum Numbers for Electrons

Shapes of Orbitals and their Types | Chemistry Skills

s Atomic Orbitals - Chemistry LibreTexts

8.3 Development of Quantum Theory – CHEM 1114 – Introduction to Chemistry
